Home » 2025 » Vol. 42, Iss. 1 » Application and performance evaluation of environmentally friendly solvent-free industrial equipment coatings in industrial equipment protection
- Research article
Application and performance evaluation of environmentally friendly solvent-free industrial equipment coatings in industrial equipment protection
- Vol. 42, Iss. 1
- Pages: 3
- - 15
- Submission received: 30/08/2024
- Revised 12/10/2024
- Accepted 20/02/2025
- Published 28/02/2025
Abstract
Environmentally friendly coatings drive systematic upgrades and value creation in industrial equipment protection. This paper combines the multiple key performance characteristics of environmentally friendly coatings in industrial equipment protection with experimental research and testing to study the optimal coating mixture ratio and actual application effects. Using modified cycloaliphatic amines as a base, solvent-free industrial equipment coatings and coatings are prepared, and comprehensive testing of surface structure, physical properties, and chemical properties is conducted to evaluate coating performance levels. Experiments show that within a curing time of 0–360 seconds, the coating viscosity remains stable, and as the temperature increases from 30°C to 90°C, the decrease in coating viscosity ranges between 0–10 Pa·s. When the mixing ratio of modified cycloaliphatic amine/epoxy resin coating is 10\%, the coating’s bond strength and adhesion are 50.46 and 16.99 MPa, respectively, with wear resistance of only 39.68 MPa. The water absorption rate and chloride penetration rate are both lower than those of other ratios. Additionally, the coating exhibits optimal hydrophobic performance in terms of contact angle and electrochemical corrosion resistance.
1 Introduction
As industrialization accelerates, corrosion issues in industrial equipment have become increasingly severe. Traditional solvent-based coatings contain up to 30% or more solvents, which have significant environmental and economic impacts [1, 2, 3]. The primary function of solvents is to dissolve film-forming agents and reduce paint viscosity, enabling the formation of a relatively perfect coating during industrial equipment protection [4, 5, 6]. After coating application, solvents evaporate into the atmosphere, with only a small amount remaining in the coating [7]. In this sense, solvents in coatings are not only a major source of environmental pollution but also a significant waste of resources. As environmental regulations become increasingly stringent, the use of solvent-based coatings is gradually being restricted. Therefore, the use of environmentally friendly solvent-free coatings in industrial equipment protection has become particularly important [8, 9, 10, 11].
Environmentally friendly solvent-free coatings are high-performance coating materials with environmental protection as their core characteristic. By reducing the use of volatile organic compounds, they achieve ecological safety for industrial equipment, ensuring excellent corrosion resistance while minimizing or eliminating negative impacts on the environment and human health [12, 13, 14, 15]. Although the development of environmentally friendly solvent-free coatings is an inevitable trend and they dominate the international coating market, solvent-based coatings, which are often highly polluting, still dominate the market in China [16, 17, 18]. The reasons for this are multifaceted: first, cost issues [19]. Second, mindset issues, as many still believe that solvent-based coatings are more reliable and stable [20]. Third, there is a matter of habit, as many companies have turned habit into standard practice and are reluctant to make changes [21]. Fourth, environmentally friendly solvent-free coatings still fall short of solvent-based coatings in certain performance aspects and require further improvement [22]. Fifth, there are issues related to production processes [23]. Therefore, it is necessary for the government to establish relevant policies to strictly restrict high-pollution coatings and support the healthy development of environmentally friendly solvent-free coatings [24, 25].
Faccini et al. [26] systematically discusses environmentally friendly corrosion-resistant polymer coatings, including the impact of corrosion on the economy and environment, the implementation of relevant regulations, consumer health protection awareness, and applications in industrial fields. Bellido-Aguilar et al. [27] introduces the application of epoxy resins in commercial fields and their negative environmental impacts, emphasizing the importance of an environmentally friendly epoxy resin industry. By preparing fully bio-based epoxy coatings, hydrophobic epoxy resins have been achieved. Kargarfard et al. [28] describes a melt-type self-layered coating based on a silicone/epoxy resin hybrid system, introducing it as an easy-to-use, environmentally friendly, and industrially applicable process, and evaluates the protective performance of silicone/epoxy resin self-layered coatings with different layered structures. Tewari et al. [29] explains innovative coating technologies for depositing thin films on solid substrates, emphasizing that these methods can precisely control coating thickness and are applied across multiple industries. Wang et al. [30] highlights the significance of UV-curable coatings with self-healing functionality synthesized from renewable resources for environmental protection and sustainable development, and synthesizes itaconic acid (IA)-glycidyl methacrylate and 2-furanic acid-glycidyl methacrylate based on itaconic acid (IA) and 2-furanic acid. Todorova, Dimitrov and Herzog [31] describes a novel self-healing coating for paper protection, designed to react with oxidation products through different mechanisms, thereby forming chemical bonds between the paper surface and the coating. Van Renterghem et al. [32] employs a solvent-free method to synthesize innovative phenoxyazine precursors with exchangeable ester functional groups using appropriate ratios of bio-based phenols, various diols, and amines. The effectiveness of this method is experimentally validated, enhancing corrosion protection for aluminum substrates. Condini et al. [33] investigated a novel solvent-free, UV-LED curable coating as a robust corrosion-resistant protection for the inner surfaces of steel pipelines. The results revealed that the UV-curable coating exhibits high barrier properties and effectively prevents liquid penetration under harsh environmental conditions.
This paper conducts experimental analysis on the coating preparation effects of solvent-free mixed coatings in spray coating processes for industrial equipment, providing an environmentally friendly coating preparation solution for industrial equipment protection. Considering factors such as protective performance, cost-effectiveness, ease of application, and adaptability, epoxy resin and modified cycloaliphatic amine were selected as the components for the mixed coating, and coatings with different ratios were prepared for industrial equipment. Multi-dimensional tests were conducted, including infrared spectroscopy and electrochemical experiments, to compare the actual performance of each coating. Based on the comparison results of surface structure curing performance, rheological properties, and other factors, the optimal coating mixture ratio was comprehensively determined to assess the application effectiveness of the industrial equipment coating.
2 Principles for selecting environmentally friendly coatings and preliminary preparations for experiments
2.1 Factors to consider when selecting environmentally friendly coatings
2.1.1 Protective and economic properties of coatings
The protective performance of coatings relates to how well structures or equipment are safeguarded during transport, storage, and in service. Some coatings may meet operational requirements but fail to protect against prolonged sunlight during transport or outdoor storage, resulting in rust. This is often because steel structures leave the factory with only one or two primer layers. To prevent rust during these stages, coatings should offer strong protection, but costs must also be considered. Ideally, the chosen system balances effective protection and economic efficiency. While lower-cost, easy-to-apply coatings may initially suffice, they often lead to increased maintenance and downtime due to frequent repairs. Therefore, investing in higher-grade coatings, improved rust removal, and thicker paint films can provide better long-term value by reducing future repair costs.
2.1.2 Good application properties of the coating
Due to the large size and weight of steel structures and industrial equipment, baking paint is not suitable for use. In addition, the paint has a short shelf life, and once mixed, it tends to dry out quickly, which can make construction difficult. On the other hand, the paint has a simpler composition, lower toxicity, and can be applied using various methods. It also has less stringent requirements in terms of construction humidity, which makes construction more convenient.
2.1.3 Pay attention to the characteristics and adaptability of the primer
For example, red lead is not suitable as a primer for certain non-ferrous metals. In addition, some primers (such as polyvinyl chloride and inorganic zinc-rich primers) have poor adhesion, so the requirements for the surface treatment of the substrate are relatively high.
2.1.4 The primer, intermediate coat, and topcoat are compatible with each other
Generally speaking, there are no major compatibility issues between paints of the same type, but compatibility between different types of paint may cause adverse effects between layers of paint. Choosing the right paint with good adhesion will yield twice the result with half the effort.
2.2 Preparation of experimental materials
2.2.1 Main raw materials and equipment
To investigate the role of environmentally friendly solvent-free industrial equipment coatings in industrial equipment protection, identify the most suitable coatings, and enhance the protective efficacy of industrial equipment, this study conducted a series of tests, observations, and analyses to characterize the protective results after preparing the corresponding experimental materials. The optimal coating mixture ratio was determined when the coating protection quality was at its best.
The primary raw materials used in this experiment included: epoxy resins (618, 6101, bisphenol F), industrial grade; curing agents (cashew shell oil-modified phenol-formaldehyde amine curing agent, modified cycloaliphatic amine curing agent, fatty amine-modified curing agent), industrial grade; epoxy diluent, zinc phosphate, mica iron oxide gray, various additives, all industrial grade. The main equipment used includes: multifunctional disperser, electronic balance, hydraulic tensile testing machine, salt spray chamber.
2.2.2 Preparation of solvent-free coatings
Preparation of component A. Component A was prepared by initially blending the epoxy resin with an epoxy diluent, followed by high-speed dispersion for 10 minutes. Subsequently, the required pigments, fillers, and various additives—including dispersing agents, leveling agents, defoaming agents, and thixotropic agents—were added to the mixture. The combined ingredients were then subjected to sand grinding until the target fineness was achieved. The resulting mixture was filtered to remove any impurities and stored for subsequent use.
Preparation of component B. Component B consisted exclusively of a modified cycloaliphatic amine, which was processed by grinding, followed by filtration to ensure purity. The prepared Component B was then reserved for further formulation steps.
2.2.3 Preparation of Industrial Equipment Coatings
The preparation of industrial equipment coatings begins with substrate selection, typically utilizing low-carbon steel plate or tinplate. Surface preparation is conducted either by sandblasting to the Sa3 standard or by manual rust removal to achieve the St3.5 grade. The coating process involves thoroughly mixing components A and B at the prescribed ratio, ensuring complete homogeneity. Application of the coating is achieved using either brush application or high-pressure airless spraying, with varying mixing ratios employed to fabricate coatings with different performance characteristics suitable for industrial environments.
2.3 Performance Testing and Characterization
A comprehensive suite of analytical and mechanical tests is employed to evaluate the performance of the coatings. Fourier Transform Infrared (FTIR) spectroscopy is utilized to characterize chemical structures, with PFEMA samples applied to dry KBr plates and analyzed over a spectral range of 5000 to 500 cm\(^{-1}\).
The morphological features of the coatings are examined using Field Emission Scanning Electron Microscopy (FESEM). This technique is particularly effective for visualizing the cross-sectional microstructure of environmentally friendly, solvent-free, modified cycloaliphatic amine/epoxy resin composite coatings prepared with different mixing ratios. Samples are mounted with conductive adhesive, sputter-coated with gold, and observed under the microscope to assess their structural integrity.
Elemental composition is assessed via Energy Dispersive Spectroscopy (EDS), providing quantitative and qualitative elemental analysis. In addition, surface chemical states and bonding environments are examined through X-ray Photoelectron Spectroscopy (XPS), which is applied to the various modified cycloaliphatic amine/epoxy resin composite coatings.
Electrochemical performance is evaluated using an electrochemical workstation, with all tests conducted in a 4.0% sodium chloride solution as specified in the testing protocols. A standard three-electrode system is employed, comprising a platinum counter electrode, a saturated silver chloride reference electrode, and the coated specimen as the working electrode. Polarization curves are recorded over a potential range from \(-\)0.50 to 0.50 V at a scan rate of 15 mV/s, while electrochemical impedance spectroscopy (EIS) measurements are performed under open-circuit conditions, spanning a frequency range of 10\(^5\) Hz to 0.001 Hz with an AC amplitude of 15 mV.
The hydrophobicity and wettability of the coatings are determined using contact angle measurements. Each sample is analyzed by dispensing a 3 \(\mu\)L water droplet and measuring the resulting contact angle. To ensure reliability, measurements are repeated at ten different positions on each sample, and the average value is reported.
Boiling water resistance is assessed according to standard protocols, whereby coated samples are immersed in boiling water and visually inspected for evidence of cracks, discoloration, or other surface defects that may indicate coating failure.
Chemical solvent resistance is investigated by immersing coated samples in solutions containing 10% H\(_2\)SO\(_4\), NaOH, and NaCl at 30\(^\circ\)C for predetermined durations. The appearance of surface phenomena such as wrinkling or rust spots is noted, as these indicate compromised protective performance of the coating.
Water absorption behavior is quantified by weighing each cured and dried sample prior to immersion in deionized water at 30\(^\circ\)C for 48 hours. After removal and thorough drying, the sample is reweighed. The water absorption rate is then calculated using the following formula: \[\label{GrindEQ__1_} W = \frac{W_{1} – W_{0}}{W_{0}} \times 100\%. \tag{1}\] Here, \(W_{0}\) and \(W_{1}\) represent the sample weights before and after immersion, respectively.
The mechanical hardness of the coatings is evaluated by the pencil scratch method. In this test, the pencil lead is shaped to a flat head and pressed against the coated surface at a 40\(^\circ\) angle. The highest pencil hardness at which no scratch occurs is taken as the coating’s hardness, with five measurements averaged for accuracy.
Coating thickness is measured in accordance with relevant standards using a micrometer at ten distinct positions, with the average thickness reported. Paint film adhesion is assessed using the cross-hatch method, again following established specifications, to determine the degree of adhesion between the coating and the substrate.
3 Performance testing and analysis of environmentally friendly solvent-free industrial equipment coatings
3.1 Analysis of the surface structure properties of the coating
3.1.1 Structural characterization of coatings
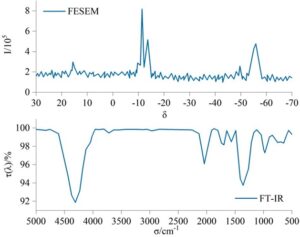
The environmental-friendly solvent-free coatings were tested and characterized in three stages: the application/spraying process, the curing process, and the rheological process over time and temperature. Figure 1 shows the infrared spectroscopy (FT-IR) and field emission scanning electron microscopy (FESEM) spectra of the environmental-friendly solvent-free industrial equipment coatings, primarily to determine whether the coating structure was successfully synthesized. The peak at 4310 cm\({}^{\mathrm{-}}\)\({}^{1}\) corresponds to the stretching vibration of the hydroxyl group (Si–OH) in the coating, while the multiple absorption peaks around 3720 cm\({}^{\mathrm{-}}\)\({}^{1}\) correspond to the stretching vibrations of C–H bonds in methyl and methylene groups. The absorption peak at 1568 cm\({}^{\mathrm{-}}\)\({}^{1}\) corresponds to the symmetric deformation vibration of the methyl group (Si–CH\(_3\)), The peak at 1359 cm\({}^{\mathrm{-}}\)\({}^{1}\) corresponds to the planar rocking vibration of methyl groups and the stretching vibration of Si–C bonds, while the absorption peaks between 1409 and 1171 cm\({}^{\mathrm{-}}\)\({}^{1}\) are attributed to the stretching vibrations of Si–O bonds. From the FESEM spectrum, peaks at chemical shifts of 15.6, -11.4, -13.6, and -55.9 show microscopic breaks, but overall calculations reveal that the branching degree of the coating is greater than 0.9. Combined with the FT-IR analysis results, this proves the successful synthesis of the composite coating.
3.1.2 Changes before and after coating curing
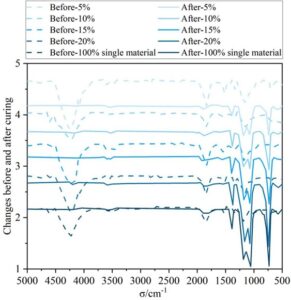
Figure 2 shows the infrared spectra of the coating material before and after curing at different mixing ratios. After blending modified cycloaliphatic amine and epoxy resin, the silanol groups and alkoxy groups in the resin undergo condensation and cross-linking reactions. As a result, after curing at room temperature, the methylene and methyl stretching vibration peaks at 1847 cm\({}^{\mathrm{-}}\)\({}^{1}\) on the alkoxy groups, as well as the hydrogen-oxygen bond stretching vibration absorption peak at 4224 cm\({}^{\mathrm{-}}\)\({}^{1}\) in the Si–OH groups, are largely eliminated. while the peak area of the Si–O stretching vibration absorption peak at 700–1500 cm\({}^{\mathrm{-}}\)\({}^{1}\) increases, indicating that the Si–OH groups have been converted into Si–O–Si bonds through condensation and cross-linking reactions. The composite material exhibits good curing performance on equipment coatings, enabling further analysis of the rheological properties of the coating material.
3.1.3 Rheological properties of coatings
Figure 3 shows the rheological properties of the blended materials on the coating. Within a curing time of 0–360 seconds, the viscosity of the composite materials with a mixing ratio of 5–20% remains stable on their respective coatings, with no significant rheological changes observed overall. For example, at a mixing ratio of 5%, the viscosity of the coating supported by the composite material remains around 10 Pa·s. This indicates that, under a fixed mixing ratio, the viscosity of the coated material is unaffected by time, providing reliable long-term protection for industrial equipment. As the temperature increases from 30\(\mathrm{{}^\circ}\)C to 90\(\mathrm{{}^\circ}\)C, the coating viscosity shows a decreasing trend, but the overall viscosity fluctuation does not exceed 10 Pa·s. Due to the significant temperature increase range, the molecular chain segments in the modified cycloaliphatic amine/epoxy resin move faster, the intermolecular forces weaken, leading to increased molecular spacing and reduced internal friction within the resin. Concurrently, the reduced entanglement density between segments decreases the flow resistance of the modified cycloaliphatic amine/epoxy resin mixture, manifesting macroscopically as a reduction in resin viscosity. However, since the temperature increase from 30\(\mathrm{{}^\circ}\)C to 90\(\mathrm{{}^\circ}\)C represents an extreme temperature change, the range of viscosity reduction falls within acceptable fluctuations. The designed environmentally friendly solvent-free industrial equipment coating exhibits good viscosity performance and can play a corresponding role in industrial equipment protection.

3.2 Analysis of coating physical properties
3.2.1 Coating bond strength and adhesion
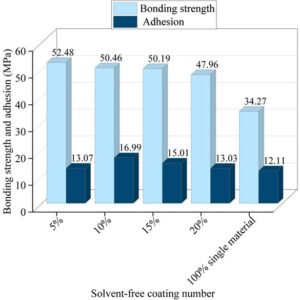
Figure 4 shows the bond strength and adhesion test results of various coatings under different mixing ratios. With a mixing ratio increment of 5%, the bond strengths of the coatings under mixing ratios ranging from 5% to 20%, as well as the coating under 100% pure epoxy resin, are 52.48, 50.46, 50.19, 47.96, and 34.27 MPa, respectively, while the adhesion strengths were 13.07, 16.99, 15.01, 13.03, and 12.11 MPa, respectively. When the solvent-free mixture ratio of the two materials reaches 10%, the bond strength reaches a high value of 50.46 MPa, while the adhesion strength reaches the highest value of 16.99 MPa. Therefore, from the perspective of bond strength and adhesion performance, the optimal modified cycloaliphatic amine/epoxy resin mixture ratio is 10%.
3.2.2 Wear resistance of coatings
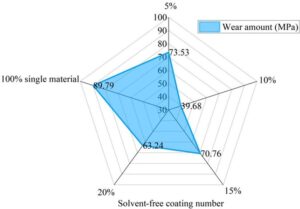
Figure 5 shows the wear volume of each coating after being scratched with a pencil of the same hardness under different mixing ratios. The average wear rates after five pencil scratches for each mixture ratio were 73.53 MPa (5%), 39.68 MPa (10%), 70.76 MPa (15%), 63.24 MPa (20%), and 89.79 MPa (100% single material). The lowest wear rate was 39.68 MPa for the 10% material mixture ratio, while the highest was 89.79 MPa for the single-material coating. Based on the comparison of wear resistance performance, the coating with a 10% modified cycloaliphatic amine/epoxy resin mixture ratio is more suitable for protecting industrial equipment.
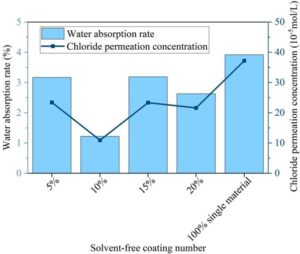
3.2.3. Coating waterproofing and chloride resistance
Figure 6 shows the water absorption rates and chloride penetration levels of various coatings at different mixing ratios. The water absorption rate and chloride resistance of the coating at the physical level determine the stability of its surface structure. In tests involving boiling water and water absorption rate, the composite environmentally friendly industrial equipment coating with a 10% mixing ratio had a water absorption rate of only 1.22% and a chloride penetration rate of only 10.93 \(\mathrm{\times}\) 10\(^5\) mol/L, indicating that the coating surface at this ratio exhibits superior waterproofing performance and chloride resistance.
3.3 Analysis of coating chemical properties
3.3.1 Water repellency of coating contact angle
Figure 7 shows the hydrophobic performance of the contact angle for each coating at different mixing ratios. After 10 tests, the average of the best contact angles from the 10 tests was taken as the final contact angle for the coating, and the hydrophobic performance of the contact angles for each coating was compared. From the perspective of contact angle, hydrophobicity rate, and spray application cycles, the industrial equipment coating at a 10% mixing ratio achieved a contact angle of 65\(\mathrm{{}^\circ}\) and a hydrophobicity rate of 92%. When water droplets come into contact with the environmentally friendly solvent-free industrial equipment coating, they tend to spread out across the coating surface, thereby meeting the superhydrophobic standard. Additionally, the industrial equipment coating at a 10% mixing ratio only requires four spray applications to maintain uniform internal structure of the material powder and achieve excellent hydrophobic performance at the chemical level, thereby reducing the risk of internal structural deformation.

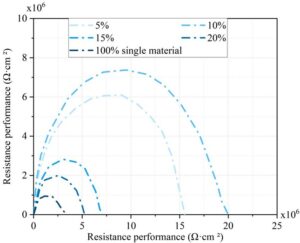
3.3.2 Electrochemical corrosion resistance of coatings
Figure 8 shows the corrosion resistance of coatings with different mixing ratios based on electrochemical tests. In the same frequency range, a larger arc radius reflects stronger corrosion resistance and greater coating density. The 10% mixing ratio exhibits the highest corrosion resistance, with an arc radius between 0\(\mathrm{\times}\)10\(^6\) and 20\(\mathrm{\times}\)10\(^6\), outperforming other ratios. These results suggest that a 10% modified cycloaliphatic amine/epoxy resin blend provides the best protective effect and reduces maintenance needs.
4 Conclusion
This paper designs a solvent-free coating for industrial equipment with multiple mixing ratios and analyzes the optimal coating mixture ratio through testing. In terms of surface structure performance, the coating has a branching degree greater than 0.9, indicating good structural curing effects. Additionally, within a curing time range of 0–360 seconds, the viscosity of coatings with various mixing ratios does not exhibit significant fluctuations over time, and viscosity fluctuations under extreme temperature changes do not exceed 10 Pa·s. In physical property testing, the coating with a 10% mixing ratio exhibits the best performance. The bond strength is 50.46 MPa, with the highest adhesion reaching 16.99 MPa. The wear rate is as low as 39.68 MPa, lower than the 73.53 MPa, 70.76 MPa, 63.24 MPa, and 89.79 MPa of other ratios. Water absorption and chloride penetration were only 1.22% and 10.93 \(\mathrm{\times}\) 10\(^5\) mol/L, respectively. In chemical performance testing, the coating at a 10% mixing ratio also demonstrated the best protective effect. The contact angle reached 65\(\mathrm{{}^\circ}\), with a hydrophobicity rate as high as 92%, and the coating required only four sprays. The maximum electrochemical corrosion protection radius was 20 \(\mathrm{\times}\) 10\(^6\).
The environmentally friendly solvent-free industrial equipment coating prepared in this study exhibits the best protective performance and economic benefits at a coating mixture ratio of 10%. Future research could explore the potential for cost reduction and efficiency improvement in the coating formulation to provide better solutions for industrial equipment protection.
References
Rao, P., & Mulky, L. (2023). Erosion‐Corrosion of Materials in Industrial Equipment: A Review. ChemElectroChem, 10(16), e202300152.
Gutoff, E. B., & Cohen, E. D. (2016). Water-and solvent-based coating technology. In Multilayer Flexible Packaging (pp. 205-234). William Andrew Publishing.
Kumar, S., Kumar, S., & Namburi, E. P. (2024). Functional Paints and Coatings. In Novel Defence Functional and Engineering Materials (NDFEM) Volume 1: Functional Materials for Defence Applications (pp. 219-246). Singapore: Springer Nature Singapore.
Jhamb, S., Liang, X., Dam-Johansen, K., & Kontogeorgis, G. M. (2020). A model-based solvent selection and design framework for organic coating formulations. Progress in Organic Coatings, 140, 105471.
Tan, Y., Zhi, Y., Gao, M., Cheng, Z., Yan, B., Nie, L., … & Hou, L. A. (2021). Influence of temperature on formaldehyde emission parameters of solvent-based coatings. Journal of Coatings Technology and Research, 18(3), 677-684.
Scotton, R. S., Guerrini, L. M., & Oliveira, M. P. (2021). Evaluation of solvent-based and UV-curing inkjet inks on the adhesion and printing quality of different aircraft surfaces coating. Progress in Organic Coatings, 158, 106389.
Durrani, T., Clapp, R., Harrison, R., & Shusterman, D. (2020). Solvent‐based paint and varnish removers: a focused toxicologic review of existing and alternative constituents. Journal of Applied Toxicology, 40(10), 1325-1341.
Langer, E., Zubielewicz, M., Królikowska, A., Komorowski, L., Krawczyk, K., Wanner, M., … & Hilt, M. (2025). Zinc-reduced anticorrosive primers—water-based versus solvent-based. Coatings, 15(1), 64.
Swartz, N. A., & Clare, T. L. (2012). Understanding the differences in film formation mechanisms of two comparable solvent based and water-borne coatings on bronze substrates by electrochemical impedance spectroscopy. Electrochimica acta, 62, 199-206.
Hazir, E., & Koc, K. H. (2019). Evaluation of wood surface coating performance using water based, solvent based and powder coating. Maderas. Ciencia y tecnología, 21(4), 467-480.
De Riccardis, M. F. (2022). Green coatings: materials, deposition processes, and applications. In Handbook of Green and Sustainable Nanotechnology: Fundamentals, Developments and Applications (pp. 1-28). Cham: Springer International Publishing.
Li, P., Chu, Z., Chen, Y., Yuan, T., & Yang, Z. (2021). One-pot and solvent-free synthesis of castor oil-based polyurethane acrylate oligomers for UV-curable coatings applications. Progress in Organic Coatings, 159, 106398.
Ozbay, S., Cengiz, U., & Erbil, H. Y. (2019). Solvent-free synthesis of a superamphiphobic surface by green chemistry. ACS Applied Polymer Materials, 1(8), 2033-2043.
Bayer, I. S. (2020). Superhydrophobic coatings from ecofriendly materials and processes: a review. Advanced Materials Interfaces, 7(13), 2000095.
Man, L., Hu, Y., Feng, Y., Zhang, C., Yuan, T., & Yang, Z. (2019). Facile synthesis of a novel bio-based methacrylate monomer derived from tung oil and its application for solvent-free thermosetting coatings. Industrial Crops and Products, 133, 348-356.
Yuan, S., Zhao, X., Jin, Z., Liu, N., Zhang, B., Wang, L., … & Hou, B. (2022). Fabrication of an environment-friendly epoxy coating with flexible superhydrophobicity and anti-corrosion performance. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 633, 127545.
Adeboye, S. A., Adebowale, A. D., Siyanbola, T. O., & Ajanaku, K. O. (2023, June). Coatings and the environment: a review of problems, progress and prospects. In IOP Conference Series: Earth and Environmental Science (Vol. 1197, No. 1, p. 012012). IOP Publishing.
Han, Z., Shi, J., Ding, C., Li, S., Cui, C., Duan, S., … & Guo, X. (2020, February). Preparation and research of brush-coated solvent-free polyurethane anti-corrosion coatings. In IOP Conference Series: Materials Science and Engineering (Vol. 772, No. 1, p. 012065). IOP Publishing.
Abolhasani, H., Farzi, G., Davoodi, A., Vakili-Azghandi, M., Das, O., & Neisiany, R. E. (2023). Development of self-healable acrylic water-based environmental-friendly coating as an alternative to chromates coatings. Progress in Organic Coatings, 176, 107402.
Ho, J., Mudraboyina, B., Spence-Elder, C., Resendes, R., Cunningham, M. F., & Jessop, P. G. (2018). Water-borne coatings that share the mechanism of action of oil-based coatings. Green Chemistry, 20(8), 1899-1905.
Zotov, A. V., Rastorguev, D. A., & Dema, R. R. (2019). Environmentally Friendly Technology for Applying Coatings with Various Functional Purposes. In IOP Conference Series: Earth and Environmental Science (Vol. 224, No. 1, p. 012038). IOP Publishing.
Dubois, A., Dubar, M., Debras, C., Hermange, K., Nivot, C., & Courtois, C. (2018). New environmentally friendly coatings for hot forging tools. Surface and Coatings Technology, 344, 342-352.
Ma, S., Wang, Y., Zheng, H., Qi, L., & Xiong, C. (2023). Environment-friendly coating with good weather resistance prepared by amorphous photonic crystals and water-dispersible resin. Optical Materials, 140, 113908.
Exarchos, D. A., Sima, A. D., Ananiadis, I., Dalla, P. A., Tragazikis, I., Kordatou, T., … & Matikas, T. E. (2022, April). Development and characterization of environmentally friendly multifunctional protective coatings. In NDE 4.0, Predictive Maintenance, and Communication and Energy Systems in a Globally Networked World (Vol. 12049, pp. 102-116). SPIE.
Wank, A., Schmengler, C., Krause, A., Müller-Roden, K., & Wessler, T. (2023). Environmentally friendly protective coatings for brake disks. Journal of Thermal Spray Technology, 32(2), 443-455.
Faccini, M., Bautista, L., Soldi, L., Escobar, A. M., Altavilla, M., Calvet, M., … & Domínguez, E. (2021). Environmentally friendly anticorrosive polymeric coatings. Applied Sciences, 11(8), 3446.
Bellido-Aguilar, D. A., Zheng, S., Huang, Y., Zeng, X., Zhang, Q., & Chen, Z. (2019). Solvent-free synthesis and hydrophobization of biobased epoxy coatings for anti-icing and anticorrosion applications. ACS Sustainable Chemistry & Engineering, 7(23), 19131-19141.
Kargarfard, N., Simon, F., Schlenstedt, K., Ulischberger, L., Voit, B., Gedan-Smolka, M., & Zimmerer, C. (2021). Self-stratifying powder coatings based on eco-friendly, solvent-free epoxy/silicone technology for simultaneous corrosion and weather protection. Progress in Organic Coatings, 161, 106443.
Tewari, K., Thapliyal, D., Bhargava, C. K., Verma, S., Mehra, A., Rana, S., … & Arya, R. K. (2024). Innovative coating methods for the industrial applications. Functional Coatings: Innovations and Challenges, 23-50.
Wang, S., Wu, Y., Dai, J., Teng, N., Peng, Y., Cao, L., & Liu, X. (2020). Making organic coatings greener: Renewable resource, solvent-free synthesis, UV curing and repairability. European Polymer Journal, 123, 109439.
Todorova, D., Dimitrov, K., & Herzog, M. (2021). Solvent free UV curable coating for paper protection. Sustainable Chemistry and Pharmacy, 24, 100543.
Van Renterghem, L., Malekkhouyan, R., Bonnaud, L., Tavernier, R., Olivier, M., & Raquez, J. M. (2024). Solvent-free coatings based on bio-sourced benzoxazines resins with healing, repair, and recycling capabilities. Progress in Organic Coatings, 189, 108316.
Condini, A., Trentalange, C., Giuliani, A., Cristoforetti, A., & Rossi, S. (2024). Advancing corrosion protection in confined spaces: a solvent-free UV LED-curable coating for steel pipelines with enhanced barrier properties and harsh environment performance. Journal of Coatings Technology and Research, 21(6), 2009-2022.
